[ad_1]
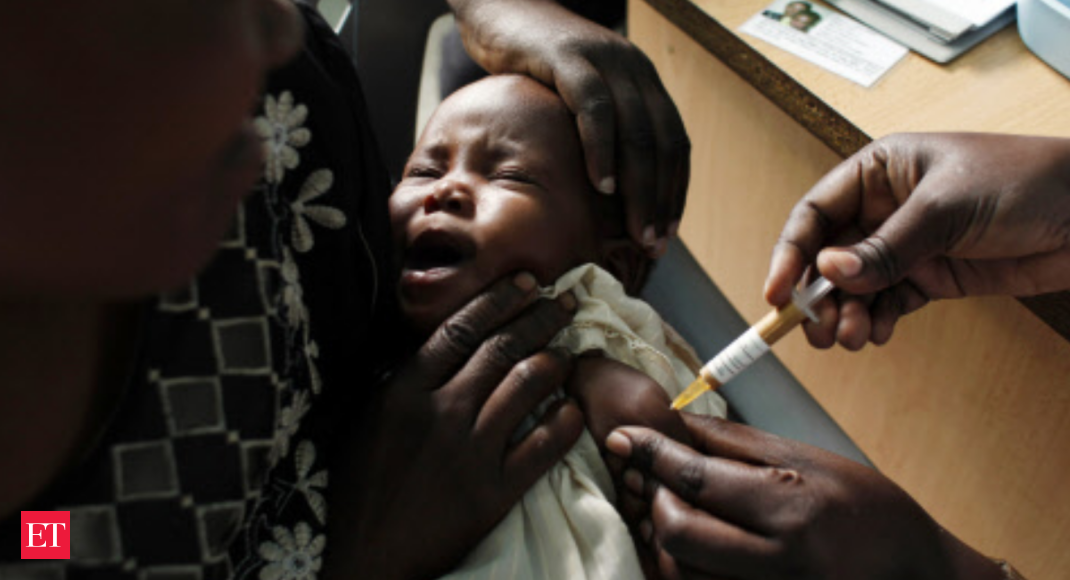
The choice adopted a assessment of a pilot programme deployed since 2019 in Ghana, Kenya and Malawi wherein greater than two million doses got of the vaccine, first made by the pharmaceutical firm GSK in 1987.
After reviewing proof from these international locations, the WHO mentioned it was “recommending the broad use of the world’s first malaria vaccine”, the company’s director normal Tedros Adhanom Ghebreyesus mentioned.
The WHO mentioned it was recommending kids in sub-Saharan Africa and in different areas with reasonable to excessive malaria transmission get 4 doses as much as the age of two.
Each two minutes, a toddler dies of malaria, the company mentioned.
Greater than half of malaria deaths worldwide are in six sub-Saharan African international locations and virtually 1 / 4 are in Nigeria alone, based on 2019 WHO figures.
Signs embody fever, complications and muscle ache, then cycles of chills, fever and sweating.
Findings from the vaccine pilot confirmed it “considerably reduces extreme malaria which is the lethal kind by 30 p.c,” mentioned Kate O’Brien, Director of WHO’s Division of Immunization, Vaccines and Biologicals.
The vaccine is “possible to ship”, she added and “it is also reaching the unreached… Two thirds of kids who do not sleep beneath a mattress internet in these international locations at the moment are benefiting from the vaccine.”
Many vaccines exist towards viruses and micro organism however this was the primary time that the WHO really helpful for broad use a vaccine towards a human parasite.
The vaccine acts towards plasmodium falciparum — one in all 5 malaria parasite species and probably the most lethal.
“From a scientific perspective it is a large breakthrough,” mentioned Pedro Alonso, Director of the WHO World Malaria Programme.
Matshidiso Moeti, the WHO regional director for Africa mentioned Wednesday’s suggestion “gives a glimmer of hope for the continent which shoulders the heaviest burden of the illness.”
The estimated value of malaria in sub-Saharan Africa is over 12 billion {dollars} a 12 months, Alonso mentioned at a information convention following the announcement.
Earlier than the newly really helpful vaccine can attain kids in want, the following step might be funding.
“That would be the subsequent main step… Then we might be arrange for scaling of doses and choices about the place the vaccine might be most helpful and the way will probably be deployed,” mentioned O’Brien.
Gavi vaccine alliance mentioned in an announcement after the WHO announcement that “international stakeholders, together with Gavi, will think about whether or not and finance a brand new malaria vaccination programme for international locations in sub-Saharan Africa.”
The struggle towards malaria obtained a lift in April when researchers from Britain’s Oxford College introduced that their Matrix-M vaccine candidate had turn into the primary to surpass the WHO’s threshold of 75-percent efficacy.
Germany’s BioNTech, which developed a coronavirus vaccine with US big Pfizer, additionally mentioned it aimed to start out trials for a malaria vaccine subsequent 12 months utilizing the identical breakthrough mRNA expertise.
The WHO additionally hopes this newest suggestion will encourage scientists to develop extra malaria vaccines.
The RTS,S/AS01 is “a primary era, actually essential one,” mentioned Alonso, “however we hope… it stimulates the sphere to search for different forms of vaccines to completement or transcend this one.”
[ad_2]
Source